In industrial effluent, Ferrate's powerful and unique chemistry can be used in the following applications:
- Removal of Heavy Metals (ex. Arsenic, Chromium)
- Disinfection (Without Disinfection Byproducts)
- Pesticide Removal
- Phosphorus Reduction
- Deodorization
- Biosolid Stabilization
- Pre-Oxidation of Organics
- Coagulation
- Algae Removal
- Biofouling and Biofilm Control
- Destruction of Emerging Contaminants (ex. EDCs, PPCPs)
- Frac Water Recycling
- Mine Waste Treatment
Industrial wastewater is a fertile field for FTT's Ferrate treatment oxidant. Industrial wastewater contaminants vary widely across such diverse industries as pulp and paper, mining, food and beverage, pharmaceutical manufacturing, electroplating, metal fabricating, aquaculture, leather tanning, oil and gas extraction, hazardous waste, and industrial farming.
There are some distinct advantages for FTT to target the industrial wastewater market. These facilities are often privately owned and governed by closely-knit boards that can quickly make decisions and will spend money to solve problems. It is FTT's strategy to form joint ventures with companies who have significant customer lists and established industry relationships in the industrial chemical sales and treatment solutions market. (See Contaminants Treatable by Ferrate)
The effectiveness of ferrate as a powerful oxidant in the entire pH range, and its use in environmental applications for the removal of abroad range of contaminants has been well documented by several researchers (Sharma, 2002; de Luca et al, 1992; White and Franklin, 1998).
Numerous possibilities exist for the use of Ferrate technology in industrial and agricultural wastes. There is scientific evidence that ferrate can effectively remove arsenic, algae, viruses, pharmaceutical waste, and other toxic heavy metals of concern (Lee et al. 2004; Kazama, 1995). A number of these treatment applications are listed below.
Mine Waste
Waste from mines can be highly toxic to the enviroment, as well as human health. Ferrate Treatment can remove cyanide, iron, manganese, aluminum, and other metals to levels that are compatible with environmental discharge.
Ferrate is used in a caustic solution (basic pH), also helps adjust the pH of acid mine drainage, which is typically very low (acidic pH).

Mine waste (Untreated vs Treated)
Frac Water Recycling
Hydraulic fracturing (fracking) is when Gas & Oil companies inject high-pressured water into wells in order to release gas and petroleum from shale for production and sale.
One of the public concerns of this process is the amount of water needed from ground water aquifers, which may be suitable for drinking water. However, if the flowback from this process can be recycled, the amount of water used and the damage to the environment can be greatly reduced.
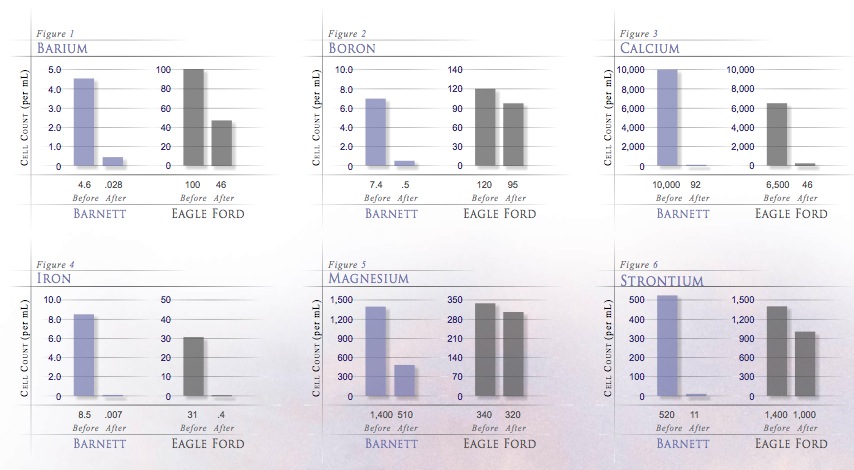
Ferrate can treat flowback and produced waters in order to be recycled and reused for well site production operations. Ferrate reduces soluble iron, barium, strontium, calcium, and manganese from these wastewaters.
Major benefits of Ferrate include:
- Disinfection of pathogens
- Break down of industrial toxins
- Removal of heavy metals
- Removal of divalent metal double salts
Industry standards for microbial contamination measure acid-producing bacteria (APB) and sulfate-reducing bacteria (SRB). Low doses of Ferrate have resulted in a complete eradication of bacteria in treated waters.
Based on recent testing (see below), treatment trains have been developed using Ferrate to either:
- Completely eradicate microbes;
- Remove more than 85 percent of undesirable divalent metals;
- Or any combination in between at lab scale.
Pharmaceutical Waste
Endocrine Disrupting Chemicals and Pharmaceuticals and Personal Care Products: Recent studies indicate the potential widespread occurrence of estrogenic compounds and other organic wastewater contaminants and their metabolites in the environment (Boyd et. al., 2003, Boyd et. al., 2004, Kolpin et. al., 2000, Snyder et. al., 2003, McLachlan, 2001, Gillette and Gillette, 1996, Daston et. al, 2003).
Estrogenic compounds include steroid hormones and their metabolic by-products, oral contraceptives, and alkylphenols. Both naturally occurring estrogenic compounds as well as estrogen-mimicking compounds may have a role in the disruption of normal endocrine functions.
Processes that can disinfect and control vector attraction along with treating the EDCs and PPCPs will be biosolids treatment processes of the future. In the Ferrate Advanced Alkaline Stabilization/Disinfection process, ferrate should react with these EDCs and PPCPs. Recently, the oxidation of hormonal estrogens, estrone (E1), 17β-estradiol (E2), and 17a-ethynylestradiol (EE2) by ferrate was studied (Hu et al. 2004).
The results suggest that the hormonal estrogens can be effectively removed by oxidation with ferrate(VI). Complete removal was obtained at a molar ratio of ferrate(VI) to estrogens ˜ three in water samples at pH 9. In summary, Ferrate is a high strength oxidant on the order of hydroxide radicals (mixed oxidants) that will oxidize sulfur and amine compounds within seconds to minutes and may also readily destroy EDCs and PPCPs.
At-Source Treatment of Effluents for Removal of Toxic Substances
Pesticides and herbicides represent significant environment contaminants that are difficult to biodegrade. Major point sources of pesticide pollution are wastewaters from agricultural industries and pesticide formulating or manufacturing plants. Effluent discharges from such sources may have pesticide contaminant levels of up to 500 mg/L (Chiron et. at., 2000).
At elevated concentrations, these compounds could be inhibitory to biological treatment process biomass at secondary treatment plants. In particular, nitrifying biological systems are considered to be sensitive processes and susceptible to inhibitory effects and shock loads.
At-source control of toxic substances prior to discharge of industrial effluent or landfill leachate to sewer can protect the biological process at the MWWTP. One potential approach in such cases is to pretreat the toxic waste at source by oxidative technologies. The pretreated effluents are more amenable to subsequent degradation by biological treatment at the industrial plant or MWWTP (Chiron et. al., 2000).
At-Source Industrial Effluent Treatment
Ferrate could be used to pretreat toxic and difficult-to-biodegrade industrial effluents. Pretreatment by ferrate may increase effluent biodegradability by downstream biological treatment.
Examples of effluents that can be considered for pretreatment by ferrate include wastewaters from textile plants, chemical manufacturing, pulp and paper production, food and beverage production, pharmaceutical plants, tanneries, explosives manufacturing, demilling operations (e.g., pink waters from demilitarizing of munitions). There are examples of oxidation of these effluent types with more conventional oxidation technologies.
At-source treatment by ferrate may be a cost-effective option compared with other chemicals owing to its strong oxidative powers. Furthermore, ferric hydroxide by-product may promote precipitation of HMW organics that may contribute to color in textile and pulp and paper effluents.
Ferrate may provide for selective decolorization of organic chromophoric constituents in industrial process waters such as textile mill effluent that are resistant to biological degradation. Complete mineralization by chemical oxidation is not likely to be economically feasible for concentrated effluents.
Treated and decolorized effluent could be considered for non-potable water recycle reuse applications. This would require filtration of suspended solids.
Treatment of Manure from Livestock Operations
Treatment of livestock manure by ferrate solution offers good potential environmental control options. In particular, large production facilities such as hog farms are particularly good candidates for at-source treatment of manure. Conversely, centralized manure treatment facilities could be installed to process waste from numerous nearby farms. The following environmental concerns can be addressed by ferrate treatment of manure.
- Removal of odor
- Deactivation of pathogens
- Destruction of organic contaminants (e.g., EDCs)
Anti-Foulant and Cleaning Agent
Ferrate solution has the potential to be used as an anti-foulant and cleaning agent for the degradation of organic foulants and for biofilm control. This could include:
- Control against nuisance biofilm (i.e. by deactivation of biomass) in pipelines and cooling towers
- Periodic cleaning of filtration media (e.g., polymeric, ceramic or stainless steel membranes, fabric filters, etc.); this would require testing of the resistance of polymeric and synthetic materials to the chemical
- Chemical cleaning is occasionally required for membrane-based treatment processes to recover filtrate flux levels; for example, membrane bioreactor (MBR) processes are susceptible to fouling and biofilm development which can limit flux over time
Algae Removal from Lagoon Effluent
Ferrate could be considered for treatment and removal of algae from facultative lagoon effluents. Concomitant with effluent treatment and disinfection, the Fe(III) by-product can promote coagulation of microscopic algae that can facilitate removal by settling or filtration. Experimental testing would be required to determine technical and economic feasibility.
Odor Control in Storage Ponds
Fe(VI) could be applied to storage ponds holding liquid waste, process water, or liquid sludge to mitigate odor problems associated with anaerobic conditions. This potential application would require case-specific investigation (i.e., economics and potential negative impacts of iron addition).
Scrubber Solution
Ferrate solution could potentially be used in scrubbers as part of off-gas odor control systems.
Odor Control
Odor control by ferrate could be applied at MWWTPs, rendering plants, and other industrial processes. Ferrate solution could also be applied to sewer lines to mitigate odor problems due to anaerobic decomposition by-products in raw sewage.