A Ferrate Laboratory Treatability Test incorporates proprietary Ferrate (Iron VI) Treatment System chemistries into a bench test under controlled conditions that are designed to meet treatment objectives specified by the client.
The chemical modification or removal of target constituents is achieved by a combination of chemical reactions that can include oxidation, disinfection, complexation, or coagulation followed by the manipulation of one or more operational parameters such as pH adjustment, sedimentation, filtration and mixing.
Case Study 1: Agricultural Stormwater
Objective
To reduce Total Phosphorus to the lowest achievable concentration, using the smallest dose of Ferrate
Results
The raw water sample received was highly colored, and had a Total Phosphorus concentration estimated to be approximately 3 ppm (3,000 ppb). A Ferrate dose of 5 ppm with ferric chloride for pH adjustment reduced Total Phosphorus to <8 ppb (the private laboratory method's analytical detection limit).
| Raw Phosphorus | Ferrate Dose | Initial pH | Ferric Chloride Dose | Treated pH | Treated Total Phosphorus | |
|---|---|---|---|---|---|---|
| Run 1 | 2.4 ppm | 5 ppm | 12.18 | 103 ppm | 4.86 | <0.008 ppm |
| Run 2 | 2.4 ppm | 5 ppm | 12.18 | 109 ppm | 5.19 | 0.21 ppm |


Case Study 2: Lead Smelter
Objective
Reduce Lead (Pb) and Cadmium (Cd) concentrations to acceptable levels
Target:
10 ppb for Lead
5 ppb for Cadmium
Results
A Lead Smelter sample with 30.3 ppb Lead and 320 ppb Cadmium was reduced to 2.62 ppb Pb and 2.74 ppb Cd with a Ferrate dose of 10 ppm and pH adjustment to 10.3 - 10.4 This is 90% of their initial concentrations and a level well below reported discharge limits of 10 ppb Pb and 5 ppb Cd to meet compliance.
Soluble concentrations for both metals were below 1 ppb.
| Parameter | RAW |
Ferrate Dose 10 ppm |
|---|---|---|
| Total Lead (Pb) - ppb | 30.3 | 2.62 |
| Total Cadmium (Cd) - ppb | 320.0 | 2.74 |
| Soluble Lead (Pb) - ppb | 1.8 | 0.19 |
| Soluble Cadmium (Cd) - ppb | 7.2 | 0.64 |
| % Removal - Lead (Pb) | --- | 91.4 |
| % Removal - Cadmium (Cd) | --- | 99.1 |
Case Study 3: Cyanide Production Facility
Objective
Reduce the Total Cyanide concentration from 6.6 ppm to <1 ppm in the plant effluent
Results
Ferrate was able to reduce the Total Cyanide concentration in the effluent to levels acceptable for environmental discharge, and could in fact reduce Cyanide levels to below detection if needed.
Case Study 4: Acid Mine Drainage
Objective
Reduce the concentrations of organics and metals including aluminum, iron, manganese, and nickel
Results
With a dose of 2 ppm, Ferrate(VI) treatment was able to destroy >98% of the organic carbon (measured as UV254 absorbance), reduce turbidity to 0.3 NTU, and residual Al & Fe were reduced by greater than 99.7%, while Ni & Mn were reduced by 65% & 43%. The acid was neutralized by Ferrate's caustic.
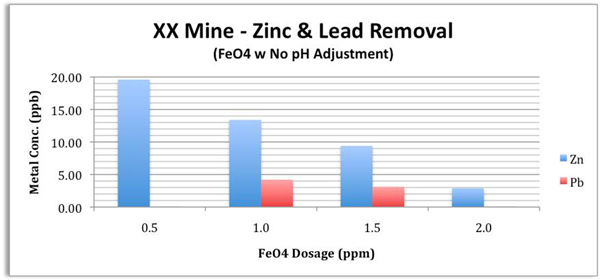
Case Study 5: Mining Wastewater
Objective
Verify Ferrate's ability to remove (via co-precipitation) toxic heavy metals
Specifically Zinc (Zn) and Lead (Pb) from the wastewater to "extremely" low levels, so that the effluent would not pose any environmental or health risk. A useful standard for comparison would be the World Health Organization's Drinking Water Standards: Zn = 3,000 ppb, and Pb = 10 ppb.
Results
Ferrate reduced Zn by > 99.8% down to < 3 ppb, and Pb by > 99.9% down to < 0.1 ppb with a dose of 2 ppm or less. This represents a water quality level equal to 1/1000 of the Zn standard and 1/100 of the Pb standard for drinking water.