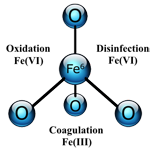
Ferrate (FeO42-)
Ferrate is a supercharged iron molecule in which iron is in the plus 6 oxidation state; it is better known as Iron(VI).
Ferrate is extremely powerful, can deliver multiple treatments from a single application, does not create disinfection byproducts, is environmentally friendly, and solves difficult treatment challenges that other oxidants can't touch.
Most importantly, Ferrate is often the least expensive and most effective treatment option.
A Laboratory Curiosity
...until now. Research scientists have been testing Ferrate for the past 50 years. Previous attempts to commercialize a Ferrate product were unsuccessful due to high synthesis, packaging and transport costs, which made Ferrate too expensive for broad industrial use.
We solved this problem by patenting a method to manufacture liquid Ferrate "onsite" so that it can be applied when its potency is highest.
More Powerful
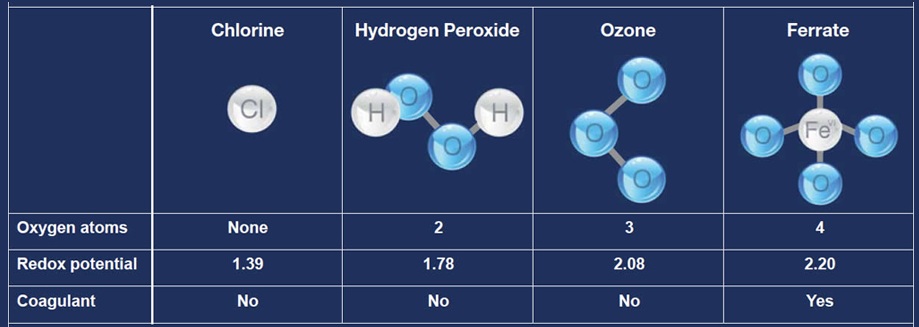
The relative strength of water treatment chemicals can be measured in volts, similar to an electrical circuit. Scientists compare the reduction - oxidation potential or "redox" potential for different compounds which is a gauge of their ability to gain or donate electrons in a chemical reaction; the higher the redox potential, the more powerful the reaction. Ferrate is the most powerful common oxidant/disinfectant for water and wastewater treatments.
Various redox potentials measured in volts are: Ferrate - 2.2, Ozone - 2.08, Hydrogen peroxide - 1.78, Permanganate - 1.68, Hypochlorite - 1.48, Perchlorate - 1.39, Chlorine - 1.36, Dissolved Oxygen - 1.23, Chlorine Dioxide - 0.95.
Multiple Treatments from a Single Dose
In a single application, Ferrate can simultaneously perform as an oxidant, coagulant, and disinfectant. Ferrate is more powerful than other oxidants such as ozone and chlorine dioxide. It can replace coagulants such as ferric chloride, alum and polymers for the removal of metals, non-metals and humic acids.
It outperforms other disinfectants such as UV, hydrogen peroxide, and chlorine and can kill many chlorine resistant organisms such as aerobic spore-formers and sulphite-reducing clostridia. Ferrate is a versatile, powerful, multi-use water and wastewater treatment technology.
No Disinfection Byproducts
Ferrate redeploys the power of chlorine for water treatment without producing disinfection byproducts. Chlorination in the presence of organics creates carcinogenic disinfection byproducts (DBPs) such as trihalomethanes (THMs), and haloacetic acids (HAAs). Ozone reacts with naturally occurring bromine to form bromates, also a human carcinogen. Switching to Ferrate from chlorine, chlorine dioxide, or ozone, precludes the formation of DBPs.
Environmentally Friendly
Ferrate is powerful, fast acting, works in small doses, and the final product of Ferrate treatment is ferric hydroxide, Iron(III), a non-toxic, environmentally benign compound. The environmental benefits of Ferrate were demonstrated during a pilot test in Pennsylvania.
The primary test objective was disinfection with neither a chlorine residual nor THM formation. The regulatory fecal coliform limit of 200 CFU/100mL was easily achieved with a Ferrate dose of 2 ppm. At the same time, freshwater aquatic toxicity tests for Daphnia showed "no observable effects" when exposed to 100% effluent through three reproductive life cycles; residual chlorine and THMs were non-detect or at trace levels in the effluent.
Least Expensive Green Technology
Ferrate is a truly unique compound that offers an economical and environmentally friendly alternative to conventional water and wastewater treatment technologies. For many applications, the capital equipment cost and aggregate operating expenses associated with Ferrate are appreciably less than other methods of treatment.
Ferrate synthesis uses commodity chemicals already found in most water and wastewater treatment plants, and a Ferrate treatment system utilizes less real estate and consumes less energy. In terms of its impact on the environment, Ferrate is a genuinely green technology that is both effective and affordable.
Unique Treatment Benefits
Ferrate has been demonstrated to treat antibiotics, hormones, pesticides, and personal care products that end up in sewers and pass through municipal treatment plants. Low doses of Ferrate have been shown to inactivate those EDCs and PPCPs. As a simultaneous oxidant, disinfectant and coagulant, one dose of Ferrate has been demonstrated to treat drinking water in Florida with color, odor, taste and residual DBP problems by simultaneously oxidizing H2S to SO4, oxidizing and co-precipitating the color-causing organics, and precluding the formation of THMs and HAAs caused by prechlorination.
Ferrate has also been shown to disinfect highly colored or turbid waters that UV light cannot penetrate, making it ideal for the restoration of wetlands as a water reuse application. In wetlands restoration, Ferrate's iron residual is an essential micronutrient for healthy plant growth.
Solves Difficult Biosolids Treatment Challenges
Ferrate has been proven to be a key ingredient in a new biosolids-to-fertilizer process. It disinfects and stabilizes biosolids to inhibit putrefaction, destroys odors, and adds iron as a vital micronutrient. Ferrate also helps bind phosphorus and nitrogen to the organic matter to create a "slow-release" fertilizer, which prolongs its availability, increases uptake in the root zone, and reduces nutrient runoff into local streams and rivers.
Synthesized Using Common Chemical Feedstocks
Ferrate is synthesized at the point of use in a patented device called a Ferrator® using caustic, sodium or calcium hypochlorite, and ferric chloride.